Injectables:
Dermal Fillers
Care by Board-Certified Dermatologists
Comprehensive Dermatology of Long Beach
Comprehensive Dermatology of Long Beach: Plump & Define with Hyaluronic Acid Fillers
Age-defying beauty meets the artistry of hyaluronic acid fillers. As time gracefully advances, our faces undergo a natural reduction in youthful volume, attributed to the diminishing production of hyaluronic acid—the essential cushioning and moisture retention agent in our skin, joints, nerves, hair, and eyes. Embrace the transformative power of our premier hyaluronic acid fillers: the Restylane® Collection of Fillers, the Juvederm® Collection of Fillers, the Revance RHA® Collection of Fillers, and Sculptra®. Safely and easily minimize wrinkles, restore facial fullness, and unlock a rejuvenated appearance with results lasting between 6 to 12 months. Trust in the FDA-approved safety of our fillers, harnessing the power of your body's natural processes. Rediscover your youthful radiance with us.
Schedule your appointment today and embark on a journey to timeless beauty. Your transformation awaits!
Comprehensive Dermatology of Long Beach:
Restylane®, JUVÉDERM® & Revance RHA® Collections of fillers - Rediscover youthful volume
At Comprehensive Dermatology of Long Beach, we offer a wide range of dermal fillers, including popular brands like Restylane®, Juvéderm®, Skinvive™ by Juvéderm®, and the Revance RHA® collections, to help you achieve your aesthetic goals. Dermal fillers are versatile injectable treatments that can address various concerns, such as smoothing fine lines and wrinkles, restoring lost volume, and enhancing facial contours.
Restylane and Juvéderm are composed of hyaluronic acid, a naturally occurring substance in the body that attracts and retains moisture, resulting in plumper, more hydrated skin. The Revance RHA collections feature resilient hyaluronic acid (RHA) technology, designed to mimic the natural movement and flexibility of facial tissue for natural-looking results.
Whether you desire subtle enhancement or dramatic rejuvenation, our experienced practitioners will create a customized treatment plan tailored to your unique needs and desired outcome. With dermal fillers at Comprehensive Dermatology of Long Beach, you can enjoy smoother, more youthful-looking skin and enhanced facial harmony.
Schedule a consultation today to discover how dermal fillers can help you achieve your aesthetic goals and regain confidence in your appearance.
Restylane® Collection Before & After


Actual patient. Results may vary. Treated with 1.5 mL Restylane® Lyft in each cheek, and 1.5 mL Restylane® Lyft in each nasolabial fold. Two weeks after treatment.
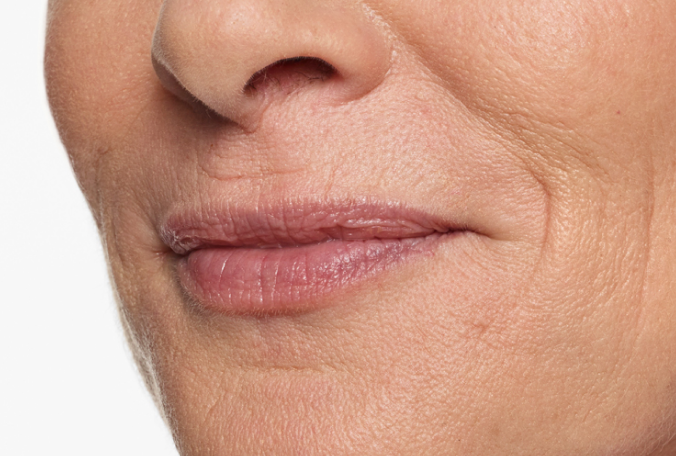
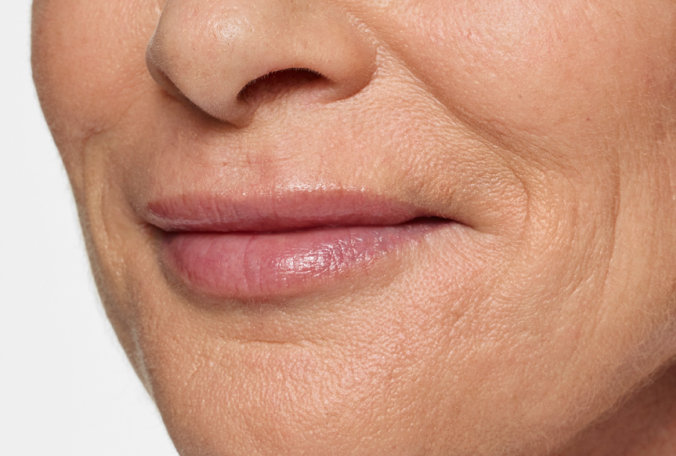
Actual patient. Results may vary. Treated with 2.3 mL of Restylane Silk in the lips and perioral (lip) lines around the mouth. Ten weeks after treatment.
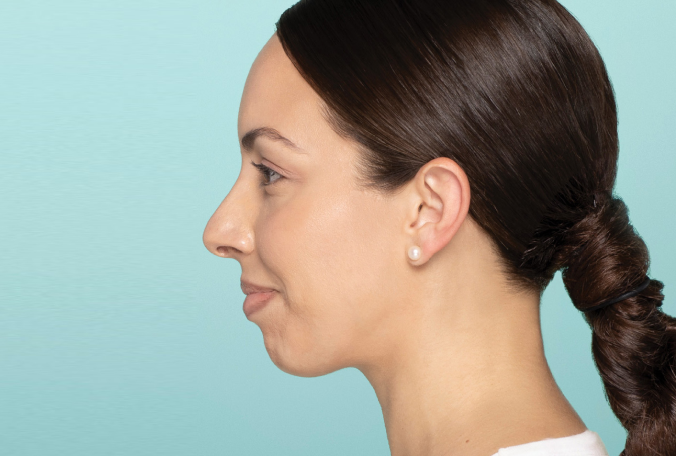
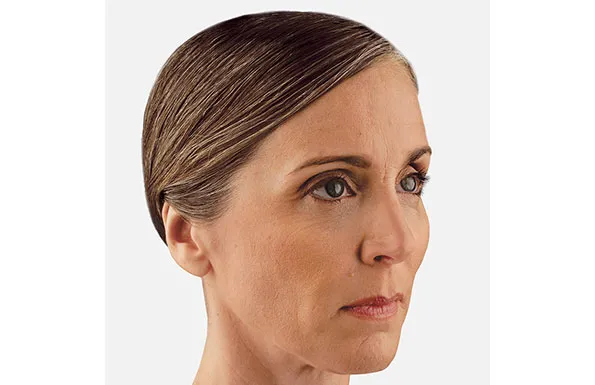
Actual patient. Results may vary. Treated with 2.6 mL of Restylane® Defyne in the chin. One week after treatment.
JUVÉDERM® Collection Before & After
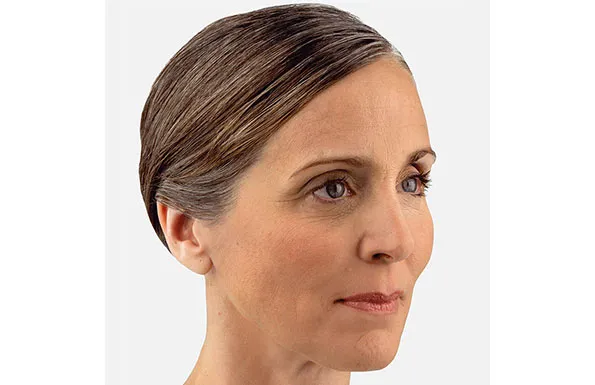
Actual patient. Results may vary. Unretouched photos taken before treatment and 1 month after treatment with 3.5 mL of JUVÉDERM® VOLUMA® XC in the cheeks.


Real patient treated with SKINVIVE™ by JUVÉDERM®. Actual results may vary.
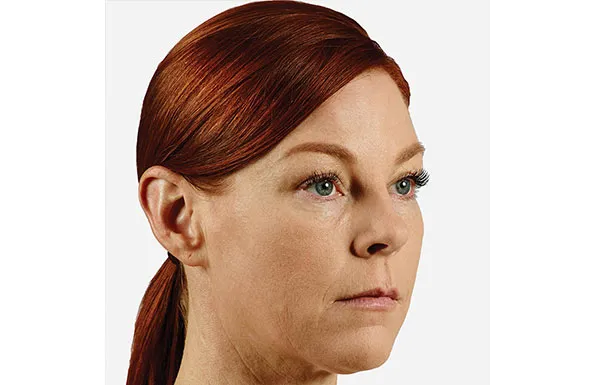
Actual patient. Results may vary. Unretouched photos taken before treatment and 1 month after treatment with 2.6 mL of JUVÉDERM® VOLUMA® XC in the cheeks.
RHA® Collection Before & After


Actual patient. Results may vary. TREATMENT AREAS: RHA® 4 1.4 mL for direct nasolabial fold correction, RHA® 4 1.6 mL for indirect nasolabial fold correction, and RHA® 4 0.6 mL in oral commissures.


Actual patient. Results may vary. TREATMENT AREAS: RHA Redensity™ 0.7 mL in perioral rhytids; RHA® 2 1.5 mL in radial cheek lines; and RHA® 3 0.8 mL for direct nasolabial fold correction, 0.2 mL for indirect nasolabial fold correction
Comprehensive Dermatology of Long Beach:
Revitalize Your Youth with Sculptra® Facial Filler
Experience lasting facial rejuvenation with Sculptra at Comprehensive Dermatology of Long Beach. FDA-approved and made with poly-L-lactic acid, this unique facial filler stimulates natural collagen production, ensuring natural-looking results that last up to 2 years.
Crafted with poly-L-lactic acid, Sculptra is a synthetic, biocompatible, and biodegradable substance widely used in reconstructive surgery for dissolvable stitches and soft tissue implants. FDA-approved for treating deep folds, marionette lines, and chin wrinkles, Sculptra stands as an exceptional anti-aging filler capable of delivering dramatic results for up to 2 years.
Discover the transformative power of Sculptra as it addresses deep folds, marionette lines, and chin wrinkles. This exceptional anti-aging filler not only ensures remarkable results but also boasts a unique approach by actively stimulating your body's natural collagen production. At Comprehensive Dermatology of Long Beach, our expert
practitioners are dedicated to providing you with a revitalized and natural-looking appearance that lasts, allowing you to embrace your timeless beauty for up to 2 years. Schedule your consultation today and let Sculptra redefine your journey to youthful radiance.
Choose Comprehensive Dermatology Of Long Beach for
Dermal Filler Treatments
Schedule Your Consultation Now!
Call us at (562) 256-9929 or click here to book online.